INTRODUCTION: Cytotoxic treatment of a primary tumor (PT) is one of the main drivers for the development of a Therapy Related Myeloid Neoplasm (TRMN). However, other intrinsic factors associated with TRMN contribute to their heterogeneous etiology. It has been suggested that around 18.5% of TRMN patients have a germline cancer mutation. On the other hand, prevalence of pre-existing clonal hematopoiesis of indeterminate potential (CHIP) is estimated in around 30% at the time of PT diagnosis, which has been associated with a worse outcome and a higher incidence of TRMN. Thus, scenarios with different etiologies must be identified for TRMN classification, differential diagnosis, and management of patients at risk.
AIM: To evaluate the relative risk factor contribution of CHIP and germline mutations to develop TRMN in patients with previous breast cancer (BC) treated with chemotherapy and/or radiotherapy.
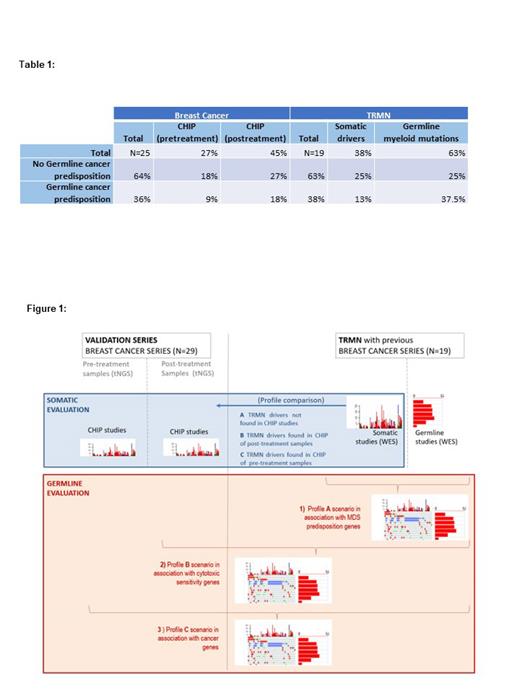
METHODOLOGY: Paired samples from bone marrow (BM) and peripheral blood (PB) of 19 TRMN patients with a previous BC have been studied by tNGS (N=7) and WES (N=12) at the time of TRMN diagnosis to describe the molecular alterations (including germline landscape and somatic drivers). BM samples were used as a source of tumoral DNA, while CD3+ T lymphocytes were isolated from PB and were used as germline sample. Variants with frequency (VAF)<30% found in BM but not in CD3+ sample were annotated as somatic. Variants with 2%>VAF<10% found in PB of BC patients were annotated as CHIP. Only pathogenic/likely pathogenic variants were considered.
RESULTS: Germline mutations in cancer predisposition genes and myeloid genes were found in 31.3% and 62.5% of TRMN patients, respectively. Myeloid somatic mutations were found in 37.5.1% of TRMN patients (Table 1). On the other hand, CHIP prevalence in longitudinal samples of BC series was found in 27.3% and 45.5% of treatment-naïve and 6-12 months after the treatment samples, respectively.
The somatic alterations found in TRMN patients were compared with CHIP in sequential blood samples of 25 BC patients. Three somatic profiles were observed (Fig.1): A) Drivers of TRMN not found in any of the BC samples might highlight that both neoplasms do not share risk factors but might involve independent germline predisposition instead. B) TRMN drivers not found in pretreatment samples but appearing in BC samples after cytotoxic therapy suggest a prominent role of treatment. C) TRMN drivers found at low frequency in the BC samples prior to the treatment might suggest that other factors different than cytotoxic therapy could be involved in TRMN development. Similar percentage of somatic drivers in TRMN patients was found in the three profiles; however, different gene frequencies were observed.
Finally, CHIP and TRMN drivers were also evaluated regarding the germline predisposition landscape. The percentage of patients with CHIP was higher in patients with no germline cancer predisposition mutations. However, a higher increase after cytotoxic treatment was observed in patients with germline predisposition (Table 1). On the other hand, TRMN patients with no germline cancer predisposition showed an increased percentage of myeloid somatic drivers (25%) in comparison with germline mutation carriers (13%). Otherwise, 37.5% of TRMN patients were carrying germline mutations in cancer predisposition and myeloid genes, suggesting low relevance of treatment in these patients.
CONCLUSION: Our results identified different etiologic scenarios in TRMN, highlighting the role of germline landscape instead of treatment. Otherwise, the percentage of somatic drivers in TRMN patients was found increased in patients with no germline cancer mutations. However, CHIP differences in pre and post treatment samples were found to be higher in BC patients with germline mutations. Deeper studies regarding the involved genes and somatic and germline associations comparing different treatment regimens are ongoing and will be further presented to discuss the contribution of different risk factors to the development of TRMN in BC patients. Understanding the risk factors contribution to TRMN development is needed for the adequate management of BC patients.
Financial support: This work was supported by a grant from the Instituto de Salud Carlos III, Ministerio de Economia y Competitividad, Spain (PI 20/00531)(Co-funded by European Regional Development Fund. ERDF, a way to build Europe) and Fundació Adey.
Disclosures
Montalban-Bravo:Takeda: Research Funding; Rigel: Research Funding. DiNardo:Novartis: Honoraria; Fogham: Honoraria; BMS: Honoraria; AbbVie/Genentech: Honoraria; Notable Labs: Honoraria; Schrödinger: Consultancy; Takeda: Honoraria; Servier: Honoraria; ImmuniOnc: Honoraria; Astellas: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal